University of Washington researchers have developed a targeted nanoparticle that kills cancer cells by simultaneously delivering chemotherapeutic drugs and activating the immune system.
Aggressive cancers that form, grow and spread quickly are notoriously difficult to treat. These cancers often become resistant to chemotherapy drugs and have some of the worst patient outcomes. New treatment strategies are desperately needed.
Immunotherapy is an increasingly common treatment that directs the patient’s own immune system to recognize and kill cancer cells. Combining immunotherapy with chemotherapy has emerged as a promising approach to circumventing drug resistance. But administering these therapies such that they reach the tumor and take effect at the same time is difficult because they are distributed, metabolized and eliminated from the body at different rates. Moreover, these treatments can have toxic side effects as a result of non-specific interactions with healthy cells.
Scientists at the University of Washington (UW) have recently developed a new nanoparticle-based drug delivery system that simultaneously delivers chemo- and immune- therapeutics directly to the tumor site, limiting harmful off-target side effects. In a paper published last November in Materials Today, they reported that their multifunctional nanoparticle can inhibit tumor growth and spread, also known as metastasis, in mouse models of triple negative breast cancer (TNBC), an exceptionally aggressive form of breast cancer with limited treatment options.

“It’s common knowledge that cancer drugs are highly toxic because they don’t just attack cancer cells, they attack all cells indiscriminately,” said senior author Miqin Zhang, a UW professor of materials science and engineering and of neurological surgery. “Using nanoparticles to selectively deliver therapeutics to where they are actually needed, i.e. the site of the tumor, will make them safer and improve their efficacy. This is the future of medicine.”
Loading sufficient amounts of both chemotherapy drugs and immune stimulating agents into a single nanoparticle, while keeping the nanoparticle exceptionally small and stable, is a formidable challenge. Nanoparticles larger than 100nm are often eliminated by the body before they can reach their target and have a difficult time getting into cells. Another challenge is synthesizing nanoparticles such that there is little variability in their size and properties, so that researchers can predict and control nanoparticle behavior in the body.
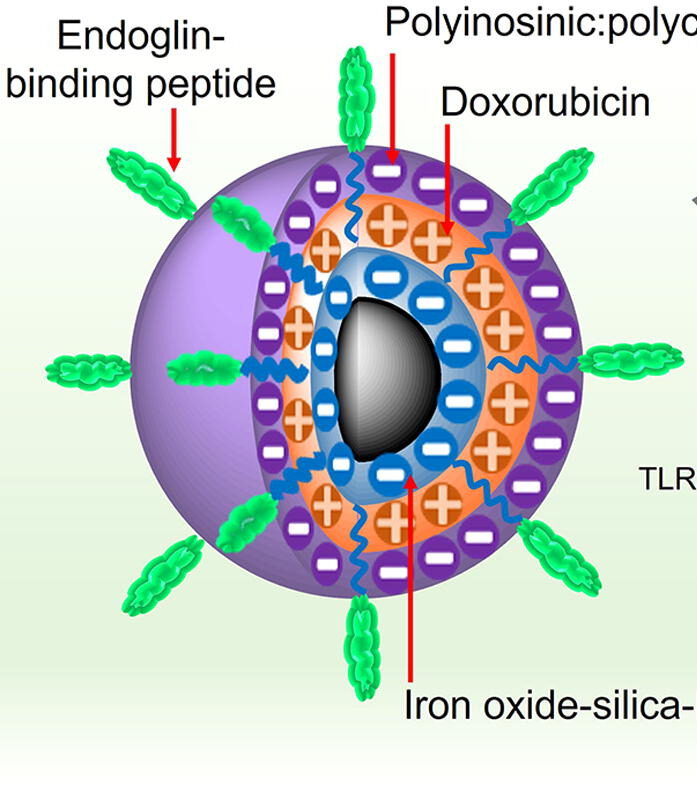
UW researchers designed the nanoparticles with their eventual use in the clinic in mind. The nanoparticle contains an iron oxide core that is biocompatible, biodegradable and detectable in magnetic resonance imaging (MRI), an especially useful tool for tracking the distribution of nanoparticles in the body. Zhang’s team used a technique known as layer-by-layer assembly to load the iron oxide nanoparticles with a tumor-targeting molecule, the potent chemotherapy drug doxorubicin (DOX) which kills cancer cells by damaging their DNA, and Poly IC, a double stranded RNA virus-mimic that stimulates the immune system. To minimize the size of the nanoparticle to just 53 nanometers, the team alternated between layers of positively charged DOX and negatively charged Poly IC, avoiding the need for additional layers of charged polymers that would further enlarge the overall size of the nanoparticle.
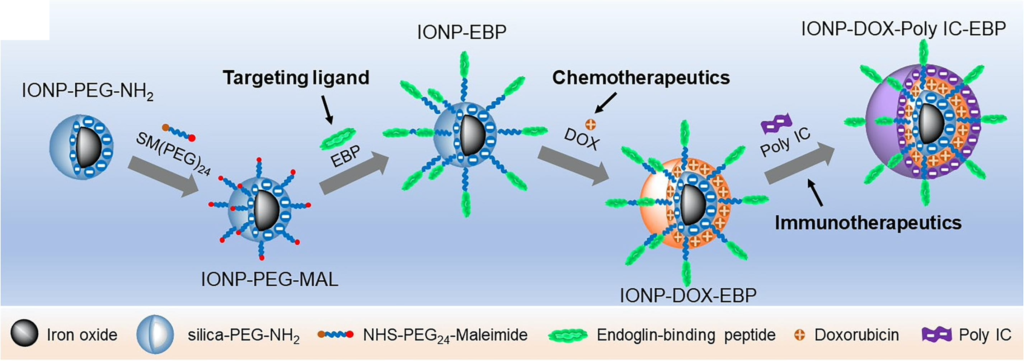
“These agents work together in a double-pronged attack on cancer cells,” said Zhang, who is also a faculty researcher with the UW Institute for Nano-Engineered Systems and the Molecular Engineering and Sciences Institute. “We deliver chemotherapeutic drugs to destroy tumors from the inside, and at the same time trigger the body’s own immune system to attack from the outside. This is the first instance in which chemotherapy and immunotherapy have been administered together in a single integrated cancer treatment.”
To limit exposure to DOX, release of DOX from the nanoparticle is pH-dependent, ensuring the drug is only released upon internalization by the target cell. In cell cultures of mouse mammary cancer cells, which mimic late stage metastatic breast cancer in humans, Zhang’s team showed that the nanoparticles are internalized by cells and that this internalization induces cell death at a level similar to that of freely administered DOX.
The team also demonstrated that their Poly IC-containing nanoparticles activate immune cells known as dendritic cells (DC), and induce the production of IL-12, a cell signaling protein that stimulates the growth and function of T cells, which are part of the more specific, adaptive immune response. Interestingly, DC uptake of nanoparticles did not result in their death, likely because DOX is not very toxic to DCs and DCs did not as readily transfer DOX into the nucleus. Researchers showed that the nanoparticles can also directly activate T cells to recognize and destroy cancer cells.
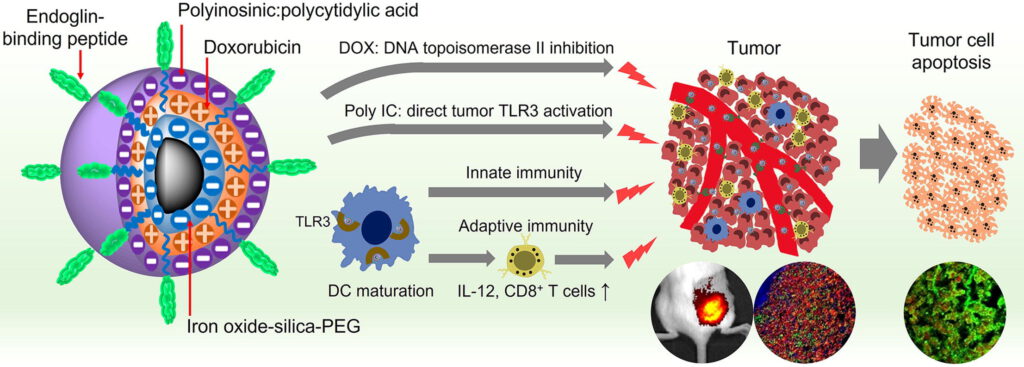
Following intravenous administration, the nanoparticle makes its way to the breast cancer tumor via endoglin-binding peptide, a small molecule that binds to endoglin, a highly expressed protein in both TNBC tumors and the blood vessels that provide nutrients to support tumor growth. The team confirmed in tumor-bearing mice that compared to administration of just DOX, their nanoparticles were highly localized to the tumor. Moreover, researchers found that delivery of DOX via nanoparticles reduced or eliminated heart damage normally triggered by DOX.
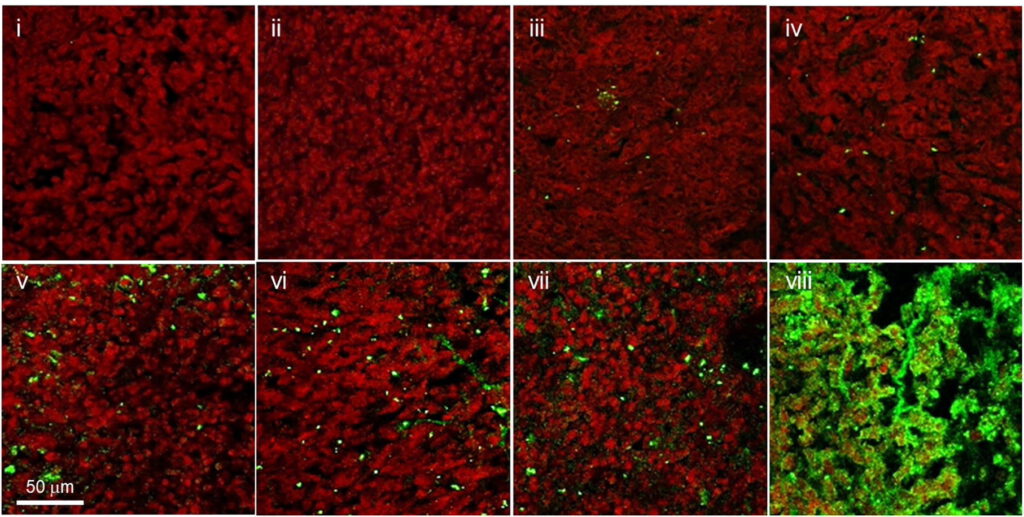
After characterizing the properties and behavior of the nanoparticles, the team evaluated the therapeutic efficacy of the nanoparticles in an aggressive and drug-resistant metastatic mouse model of TNBC. They found that five doses of their treatment inhibited tumor growth, delayed metastasis, and extended survival compared to injection of DOX alone. Zhang is hopeful that with these promising results, they can begin to think about translating this treatment to the clinic.
“I’m excited by the potential for these nanoparticles be used in patients,” said Zhang. “In the past year, we saw lipid nanoparticles used in the context of the Moderna and Pfizer COVID-19 vaccines. I think with their overwhelming success in the clinic, we will see nanoparticles used more and more to target therapeutics and treat disease.”
Co-authors on the paper are Qingxin Mu, Guanyou Lin, Mike Jeon, Hui Wang, Fei-Chien Chang, and Richard A. Revia in the UW Department of Materials Science & Engineering and John Yu in the Department of Neurosurgery at Cedars Sinai Medical Center. The research was funded by the National Institutes of Health.
For more information, contact Zhang at mzhang@uw.edu.